Il-prodotti tagħna daħlu fil-lista tar-Renju Unit ta 'apparati dijanjostiċi invitro tal-koronavirus eżentati!
Tista' tiċċekkja l-lista fuq il-websajt tad-Dipartiment tas-Saħħa tar-Renju Unit: https://www.gov.uk/.../medical-devices-regulations-2002... Jekk għandek bżonn tixtri l-prodotti tagħna, tista' tikkuntattjana fuq kwalunkwe ħin!
Rapport tal-Ittestjar u Analiżi in Silico għal Test Rapidu tal-Antiġen StrongStep® SARS-CoV-2 fuq Varjanti SARS-CoV-2 differenti
SARS-CoV-2 issa evolva diversi mutazzjonijiet b'konsegwenzi serji, xi wħud bħal B.1.1.7,B.1.351,B.1.2,B.1.1.28,B.1.617,Inkluż ir-razza mutant omicron (B1.1.529) irrappurtat fl-aħħar jiem.Bħala manifattur tar-reaġent IVD, aħna dejjem nagħtu attenzjoni għall-iżvilupp ta 'avvenimenti rilevanti, iċċekkja l-bidliet tal-aċidi amminiċi rilevanti u nevalwaw l-impatt possibbli ta' mutazzjonijiet fuq ir-reaġenti.
StrongStep® SARS-CoV-2 Antigen Rapid Test Daħħal il-lista komuni tal-UE tal-iġjene u s-sikurezza tal-ikel
StrongStep® SARS-CoV-2 Antigen Rapid Test Daħħal il-lista komuni tal-UE tal-iġjene u s-sikurezza tal-ikel, li hija waħda mill-ftit manifatturi li għandha sensittività ta '100% meta l-valur CT ikun inqas minn 25%.
StrongStep® SARS-CoV-2 Antigen Rapid Test inkluż fil-lista ta 'evalwazzjoni FIND
StrongStep® SARS-CoV-2 Antigen Rapid Test inkluż fil-lista ta' evalwazzjoni FIND.Il-Fondazzjoni għal Dijanjostiċi Ġodda Innovattivi (FIND), hija organizzazzjoni li tispeċjalizza fl-evalwazzjoni tal-prestazzjoni ta 'kits f'kooperazzjoni strateġika mal-WHO.
Dikjarazzjoni dwar viruses varjanti
L-analiżi tal-allinjament tas-sekwenza wriet li s-sit tal-mutazzjoni tal-varjant SARS-CoV-2 osservat fir-Renju Unit, l-Afrika t'Isfel u l-Indja kollha mhumiex fir-reġjun tad-disinn tal-primer u s-sonda bħalissa.StrongStep® Novel Coronavirus (SARS-CoV-2) Multiplex Real-Time PCR Kit (skoperta għal tliet ġeni) jista 'jkopri u jiskopri razez mutanti ( murija fit-tabella li ġejja) mingħajr ma jaffettwa l-prestazzjoni fil-preżent.Minħabba li m'hemm l-ebda bidla fir-reġjun tas-sekwenza ta 'skoperta.
Fil-qosor tar-Rapport ta' Evalwazzjoni minn Istitut Differenti dwar it-Test Rapidu tal-Antiġen StrongStep® SARS-CoV-2
We have received many certificate or EUA from different countries, such as United Kingdom, Singapore, Brazil, South Africa, Malaysia,Indonesia,Philippine, Argentine, Guatemala and so on. Also, we have send our products to many institue for evaluation, below are the summary of some data. Please contact us via guangming@limingbio.com if you need full artical of following report.
Tajlandja FDA COVID 19 ATK 2021 T6400429
Riċentement, it-Test Rapidu tal-Antiġen StrongStep® SARS-CoV-2 prodott minn Nanjing Liming Bio-products Co. Ltd kiseb b'suċċess iċ-ċertifikat tal-FDA tat-Tajlandja (numru ta 'reġistrazzjoni T 6400429,T 6400430,T 6400431,T 6400432), u issa ġie approvat biex jidħol fis-suq tat-Tajlandja.
StrongStep® SARS-CoV-2 Antigen Rapid Test kiseb il-verifika tal-prestazzjoni ta 'Paul-Ehrlich-Institut (PEI) fil-Ġermanja!
Riċentement, ir-reaġent ta 'skoperta ta' l-antiġenu Novil Coronavirus (SARS-CoV-2) ta 'Nanjing LimingBio "StrongStep® SARS-CoV-2 Antigen Rapid Test" kiseb il-verifika tal-prestazzjoni ta' Paul-Ehrlich-Institut (PEI*) fil-Ġermanja, dan il-prodott ġie iċċertifikat mill-Aġenzija Federali Ġermaniża għall-Amministrazzjoni tal-Mediċini u l-Apparat Mediku (BfArM).LimingBio sar wieħed mill-ftit manifatturi fiċ-Ċina li kiseb iċ-ċertifikazzjoni doppja ta 'BfArM + PEI fil-Ġermanja.It-test rapidu tal-antiġen tal-Liming Bio għadda miċ-ċertifikazzjoni awtorevoli tal-Ministeru tas-Saħħa ta 'ħafna pajjiżi, li juri bis-sħiħ il-prestazzjoni eċċellenti tal-kit.
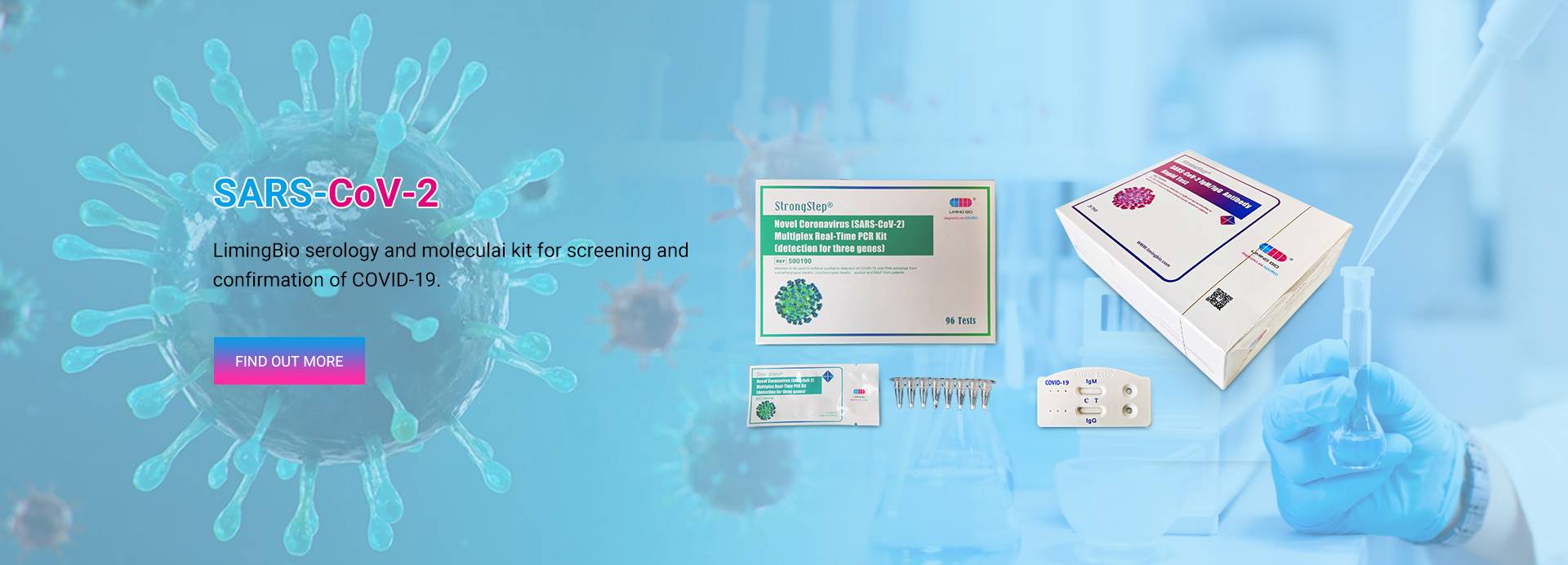
Test Rapidu tal-Antiġen tas-SARS-CoV-2
StrongStep® SARS-CoV-2 Antigen Rapid Test huwa test immunokromatografiku rapidu għall-iskoperta ta 'antiġenu COVID-19 għall-virus SARS-CoV-2 fil-griżmejn uman/tampun nasofarinġeali.
Kit PCR Multiplex Real-Time tal-Koronavirus Ġdid (SARS-CoV-2).
Dan il-kit PCR sensittiv ħafna u lest għall-użu huwa disponibbli f'format lajofilizzat (proċess ta 'tnixxif bil-friża) għal ħażna fit-tul.Il-kit jista 'jiġi ttrasportat u maħżun f'temperatura tal-kamra u huwa stabbli għal sena.
L-AĦĦAR PRODOTTI TAGĦNA
FUQNA
Nanjing Liming Bio-products Co., Ltd mwaqqfa fl-2001, il-kumpanija tagħna kienet speċjalizzata fl-iżvilupp, il-manifattura u l-kummerċjalizzazzjoni ta 'testijiet rapidi għal mard infettiv speċjalment STDs.Minbarra l-ISO13485, kważi l-prodotti kollha tagħna huma mmarkati CE u approvati CFDA.Il-prodotti tagħna wrew prestazzjoni simili meta mqabbla ma 'metodi oħra (inkluż PCR jew kultura) li jieħdu ħafna ħin u jiswew ħafna flus.Bl-użu tat-testijiet rapidi tagħna, jew il-pazjent jew il-professjonisti tal-kura tas-saħħa jistgħu jiffrankaw ħafna ħin għall-istennija għax jeħtieġ biss 10 minuti.



















1人份抗原卡实物图唾液版1_00_副本-300x216.png)


















